What is Conductivity?
Conductivity is the ability of a material to transfer an electric charge from one point to another. To transfer current, charged particles must be present in the solution. It is a standard practice to measure conductivity in aqueous solutions as different salts, acids, and bases dissolved in water act as electrolytes and provides ions. How well a solution conducts electricity is measured by the term conductivity. Ohm’s Law provides the basic idea of Conductivity.
E = I • R
where E = applied voltage between two “plates”
I= electrical current
R=Resistance of the conductor.
From the above equation, conductivity can be defined as the reciprocal of resistance of a solution between two electrodes and it is expressed as:
G = I/R
The units used for conductivity are Mhos or more commonly used Siemens. Mhos and Siemens can be used interchangeably.
However, to compensate for variations in the electrode dimensions, standardized measurements are expressed in specific conductivity units. By multiplying the measured conductivity (G) as mentioned above by the electrode’s cell constant (L/A); Specific conductivity is calculated. Hence, Specific conductivity (C) is given by
C = G x (L/A)
where L=the length of the liquid column between the electrodes
A= area of the electrodes
Conductivity meters use this equation to calculate the conductivity of a solution and display the results.
Resistance is the inverse of conductance. Materials that are in the liquid phase and conduct electrical current are called electrolytes.
Application of Conductivity Measurement
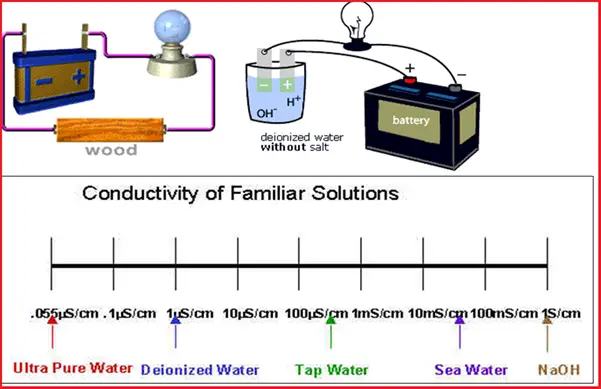
Conductivity measurement is widely used for quality control purposes. For monitoring and controlling of used water, conductivity is one of the important, cost-effective, and stable parameters. That is the reason conductivity measurement has found numerous industrial applications.
The major areas where conductivity is important are listed below:
- Treatment of Water Quality
- RO systems; Conductivity measurement of pure water.
- Desalination industry.
- Boiler feedwater protection.
- Water towers.
- Hardness protection in laundries.
- Salinity Testing.
- Condensate and Steam quality protection.
- Wastewater treatment.
- TDS Testing.
- Concentration measurements and control
- Finding the concentration of various acids like Hydrochloric acid, Phosphate, Ammonium and ammonia gas, Nitric acid, Sulphuric Acid, etc.
- Conductivity measurement can also be helpful in analyzing Carbon dioxide and Sulphuric dioxide in water.
Refer to the following figure (Fig. 1) that lists all the applications of conductivity measurements.


Measurement of Conductivity
The devices that are used to measure conductivity are called Conductivity Analyzers or Conductivity meters. There are two ways in which the conductivity in liquids or slurries is measured:
- Contacting (or Electrode) Measurement.
- Toroidal (Inductive or Electrodeless) Measurement.
Contacting or Electrode Type Measurement (Fig. 3):
- The two-electrode methodology uses two opposing electrodes.
- The anode is supplied with a known current, which is picked up by the cathode when placed in an electrolyte.
- The amount of current picked up by the cathode is dependent upon the conductance of the electrolyte.
Probe/Cell Constant of Conductivity Meter:
The cell constant is the distance between the electrodes divided by the area of the electrodes.
K (cm-1) = Distance between electrodes (cm) / Area of electrodes (cm)
The smaller the cell constant the higher the signal that will be returned to the meter.
Low conductivity solutions use a small cell constant and high conductivity solutions will use a larger cell constant sensor.
Two Electrode Methodology:
Its main drawback is:-
- The sensor is susceptible to coating and corrosion, which drastically lowers the reading.
- In strongly conductive solutions there can also be polarization effects, which results in non-linearity of the measurement.
The Four Electrode Methodology for Conductivity measurement:
- It utilizes the same two-electrode measuring scheme, but it also includes an additional two-electrode system to act as a reference point for the measuring circuit, for use in applications where light coats from the process can occur.
- The minimum range for this type of electrode is approximately 5000 micro S/cm.
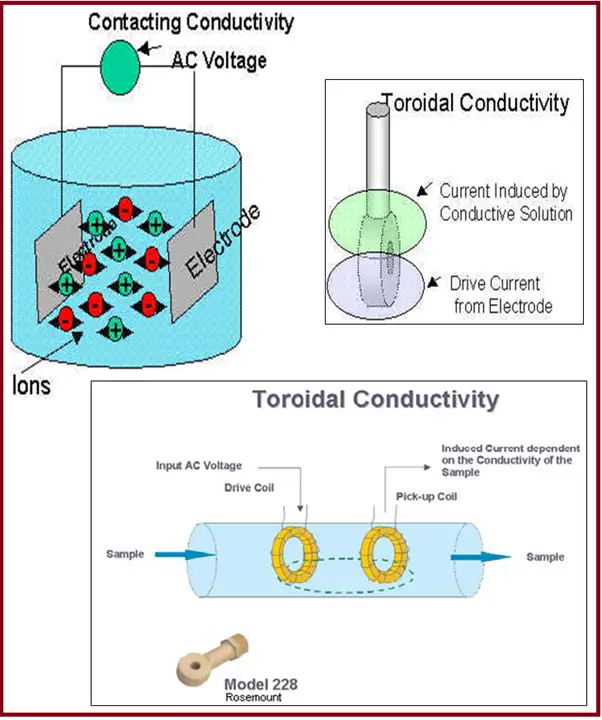
Toroidal Type Measurement (Fig. 3):
- Toroidal conductivity measurement is made by passing an AC current through a Toroidal drive coil, which induces a current in the electrolyte solution.
- This induced solution current, in turn, induces a current in a second toroidal coil, called the pick-up toroid.
- The amount of current induced in the pick-up toroid is proportional to the solution conductivity.
Advantages of Toroidal Type Conductivity:
- The toroidal coils are not in contact with the solution.
- The insertion-style Toroidal sensor can be completely coated by a solid OR oily contaminant in the process, with essentially NO lowering of the reading until the coating displaces a significant volume of the surrounding liquid.
- The polymeric material housing the Toroids can be chosen to be compatible with corrosive solutions,
Drawbacks of Toroidal Type Conductivity Measurement :
- It lacks the sensitivity of contacting measurement.
- Toroidal sensors are also typically larger than contact sensors, and the solution current induced by the Toroid occupies a volume around the sensor. Hence, Toroidal sensors need to be mounted in a larger pipe.
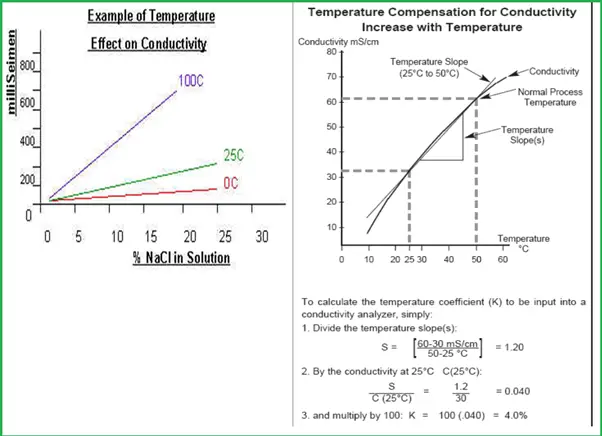
Temperature Effects on Conductivity Measurement
- Temperature does have a significant effect on the conductivity of solutions.
- The same solution concentration at different temperatures has a drastically different conductivity.
- In order to measure the conductivity temperature effect must be compensated.
For Temperature, compensation follows the steps shown in Fig. 4
The temperature coefficients of the following electrolytes generally fall in the ranges shown below:
- Acids 0 – 1.6%/°C
- Bases 8 – 2.2%/°C
- Salts 2 – 3.0%/°C
- Freshwater 0%/°C
Calibration of Conductivity Analyzers
Moderate to High Range Measurements:
- For conductivity measurements in excess of 100 μS/cm, a conductivity standard may be used to calibrate a conductivity loop.
- The conductivity measurement may also be calibrated using grab sample standardization.
- Care must be taken that the correct temperature coefficient is being used in both the online instrument and the referee instrument to avoid discrepancies based on temperature compensation errors.
High Purity Water Measurements
- Conductivity samples below 100 μS/cm are highly susceptible to contamination by trace contaminants in containers and by CO2 in the air. As a result, calibration with a conventional standard is not advisable.
- Many conductivity instruments designed for high-purity water measurements include a calibration routine for entering the constant of the conductivity sensor.
- The conductivity sensor used with this kind of instrument must have its sensor constant accurately measured using a conductivity standard in a higher range. Once the sensor constant is entered into the instrument, the conductivity loop is calibrated.
- A second method is to calibrate the online instrument to a suitably calibrated, reference instrument in a closed flow loop.
Advantages of Conductivity Measurement
- Conductivity offers Fast, Reliable, Nondestructive, Inexpensive &
- Durable means of measuring the ionic content of a sample.
- Reliability and repeatability are excellent.
Disadvantages of Conductivity Measurement
- The principle drawback of conductivity is that it is a nonspecific
- Measurement – it cannot distinguish between different types of ions,
- giving instead a reading proportional to the combined effect of all
- ions present. Therefore it must be applied with some pre-knowledge
- of the solution composition OR used in relatively pure (single solute) solutions to be successful.
Factors influencing the conductivity measurement of Conductivity Meters
The correctness of conductivity measurements can be influenced by various factors:
- Geometry
- Polarisation
- Cable resistance and capacitance
- Frequency change
- Contamination
- Temperature
Further Studies for Conductivity measurement:
Refer to the following documents to know more about conductivity and the measurement of conductivity:





Mr. Dey,
learnt from google.
The question is :- How to measure conductivity of a particular length of MS pipe used for Diesel transfer .