The important characteristic of crude oil is governed by the properties of pure hydrocarbon in terms of viscosity, density, and boiling point curves. These properties are very important for designing almost every type of equipment in the industry as well as these properties are also indicative of the quality of the product as well as the feed.
Important Characterization Properties
Below are the main characterizations parameters used in the refinery,
- API gravity
- Watson Characterization factor
- Viscosity
- Sulfur content
- True boiling point (TBP) curve
- Pour point
- Flash and fire point
- ASTM distillation curve
- Octane number
What is API gravity?
API gravity is mainly used to define heavier and lighter crude petroleum products. It is a measure of the density of the crude. The API gravity is formulated as
oAPI (API Gravity) = 141.5/S.G– 131.5
Here, S.G means specific gravity is measured at a temperature of 60 oF. At the same time, specific gravity can be calculated from a known API gravity.
An oil of S.G 1 has an API gravity of 10, i,e 10 oAPI. So, API gravity is inversely proportional to the specific gravity. So, the higher the values of the API gravity of an oil lower will be its specific gravity. Thus the oil will be lighter and vice-versa. As far as crude oil is concerned, a lighter API gravity value is more important for lighter crude oil than heavier crude oil as more amount of gas fraction, naphtha, and gas oils can be achieved from the lighter crude oil than with heavier crude oil. So, crude oil with higher API gravity is expensive to procure due to its good quality.
What is Watson’s characterization factor?
The Watson characterization factor is usually expressed as
K = (TB)1/3/S.G (at 15’C)
Here,
- S.G. is specific gravity.
- TB is the volume or average boiling point in degrees R (Rankine).
Watson’s characterization factor varies between 10.5 and 12.9 for various crude components. A k factor of 12.9 indicates highly paraffinic crude. On the other hand factor of 10.5 indicates highly naphthenic crude. Therefore, the Watson characterization factor can be used to measure the aromaticity and paraffinic of the crude.
What is Sulfur content?
Since crude oil is excavated from reservoirs, sulfur is present in the crude oil. Organic and inorganic sulfur both usually exist in crude oil. Based on the sulfur content (less than 0.5% wt) crude oil is classified into two categories. One is sour crude which contains more sulfur and the other is sweet crude which contains low sulfur. The normal range of sulfur content of crude oil is 0.5 – 5 wt %. It is observed that almost 80 % of the total crude oil across the globe is sour.
As we know the presence of sulfur is responsible for SO2 emission which is not desirable as well as for catalytic reactions sulfur reduces the efficiency of the catalyst. There are several hydro-treating operations conducted in the refineries to remove sulfur from crude oil. Presently, India is heading towards the generation of diesel with Euro III standards that indicate that the maximum sulfur content is about 500 ppm in the product. This indicates that large quantities of inorganic sulfur need to be removed from the fuel. Typically, inorganic sulfur that is normally found in various intermediate product streams is removed by the hydrogenation process using hydrogen as hydrogen sulfide.
What is True boiling Point (TBP)/ASTM distillation curves?
The TBP/ASTM distillation curve is the most important property of the crude and intermediate product streams. These TBP & Distillation curves are determined at atmospheric pressure. The boiling points of different volume fractions are determined. However, the main difference between True Boiling Point & ASTM distillation curve is that the TBP curve is generated using a batch distillation setup containing less than 100 trays with a higher reflux ratio while the ASTM distillation curve is plotted in a single-stage apparatus and the absence of reflux. Therefore, the TBP curve does indicate a good separation of the different components while ASTM does not indicate a good separation of a different component.
What is the Viscosity of Crude Oil?
Flow properties of refinery streams are measured by viscosity. The unit that is used to define viscosity is centistokes or say bolt seconds or redwood seconds. Normally, the measurements of viscosity are carried out at temperatures of 100 oF and 210 oF. For heavy products obtained from crude oil, viscosity is a very crucial property to measure as well as viscosity plays an important characterization property in the blending units, especially for a heavy product e.g. bunkers fuel.
What are Flash Point and Fire Point?
Flash and fire points are important characterization properties that are related to the process safety and transmission of refinery units. Flashpoint is the lowest temperature at which the crude oil flashes into a vapor that is able to ignite on exposure to an open flame. The flashpoint is an indication of the combustibility of a liquid. Flashpoint is normally measured in the “open cup” or “Closed Cup” apparatus. Some examples of flashpoints are as below,
- automotive gasoline, −43 °C (−45 °F)
- ethyl alcohol, 13 °C (55 °F)
- automotive diesel fuel, 38 °C (100 °F)
- kerosene, 42–72 °C (108–162 °F)
- home heating oil, 52–96 °C (126–205 °F)
- SAE 10W-30 motor oil, 216 °C (421 °F)
The fire point of crude oil is the temperature above the flashpoint where the crude oil produces sufficient vapor to catch fire.
Pour point
The pour point is the temperature below which crude oil will not flow; i.e, it will become plastic. During the cooling of a petroleum product, a cloudy appearance occurs at a specific temperature. This temperature is called the cloud point. On further reduction of the temperature of the product, the product stops the flow. Both pour and cloud points are important characterization properties of the refinery product streams as far as heavier components are concerned. For heavier fractions, they are specified in a certain range & achieved by blending proper amounts of lighter intermediate components. Click here to explore more about the significance of Pour Point.
What is an Octane number?
The octane number is an important characterization property used to define many intermediate streams that undergo blending to produce gasoline, diesel, etc. It is often called the Antiknock Rating which is used to check the ability of a fuel to prevent knocking during the ignition of a mixture with air in the cylinder of a combustion engine. The knocking tendency of a fuel is defined as the maximum compression ratio of the internal-combustion engine at which the knock occurs. Therefore, high-quality automotive gasoline tends to knock the internal-combustion engine at higher compression ratios and vice versa. However, for comparative measurement purposes, one needs to have a pure component that has a known compression ratio. Iso-octane is eventually considered as the yardstick for octane number comparison. If iso-octane is given an octane number of 100 then n-heptanes is given a scale of 0. So, the octane number of a fuel is defined as an equivalent mixture of iso-octane and n-heptane that provides the same compression ratio in an internal-combustion engine. Thus an octane number of 80 signifies that the fuel is equivalent to the performance characteristics in a combustion engine fed with 80 (vol % ) of iso-octane and 20 (vol%) of n-heptane.
Octane numbers are much related to the reforming, alkylation & isomerization processes of the refining industry. The above processes do the reactive transformation to produce side-chain paraffin that possesses high octane number compared to the feed component which does not contain higher quantities of straight-chain paraffin and non-aromatics that are called naphthenes.
Crude chemistry
Generally, Crude oil contains 84 – 87 wt % of carbon (C) , 10 – 14 % of hydrogen(H), 0 .5– 5 wt % of sulfur(S) , 0 – 2 wt % of oxygen(O) , 0 – 0.6 wt % of nitrogen(N) and some metals like copper, lead, sodium, zinc, vanadium, etc ranging from 0 – 100 ppm. It is very important to understand thoroughly the basics of crude chemistry.
Depending on chemical analysis and the existence of various functional groups like –OH,-CH3, =CH2, etc refinery crude can be classified into five main categories as mentioned below,
Paraffins: Paraffin rates the alkanes such as methane (CH4), ethane (C2H6), propane (C3H8), n-butane, iso-butane, n-pentane, and iso-pentane. The below compounds are primarily obtained as a gas fraction from the CDU (crude distillation unit).
Olefins: Basically, it is alkenes such as ethylene (C2H4), propylene (C3H6), and butylenes (C4H8) are highly unstable and susceptible to a chemical reactions. They are not obtained in a sufficient amount in crude oil but are encountered in a few refinery processes such as alkylation.
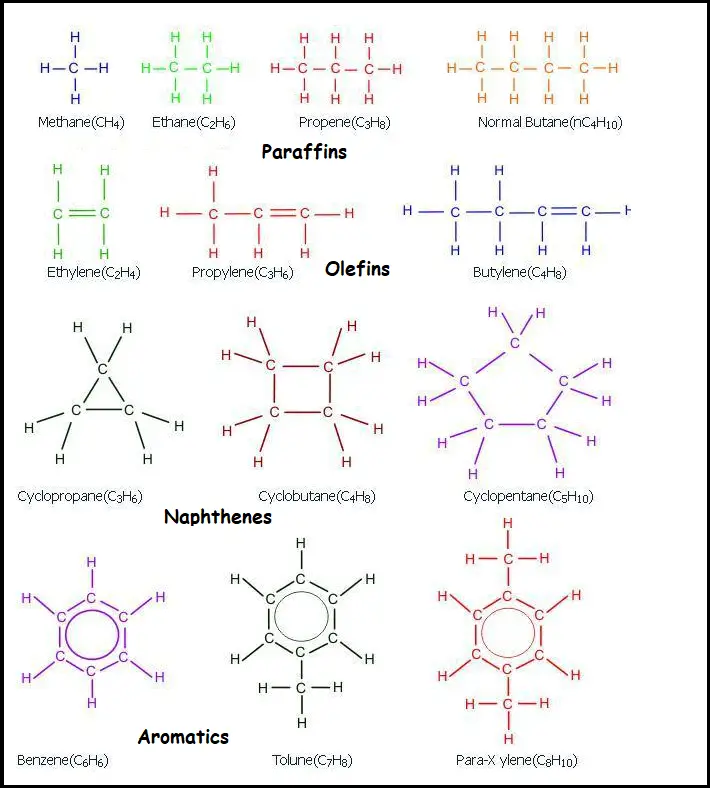
Naphthenes: Naphthenes are the cyclo-alkanes such as cyclo-propane, and methylcyclohexane present in crude oil. Naphthenes are not aromatic and so it does not possess a higher octane number. Therefore, during the reforming reaction, naphthenes are encountered to produce aromatics that possess higher octane numbers compared to naphthenes.
Aromatics: It is the chemical compound that consists of one or more rings with delocalized double bond pi electrons) such as benzene, toluene o/m/p-xylene. Aromatic compounds are available in crude oil. The aromatic compound possesses a higher octane number and it is the objective to maximize its quantity in a refinery.
Naphthalene: It is a polynuclear aromatic consisting of two or three or more aromatic rings.
Click here to explore more about Crude Oils
Online Video Courses on Crude Oils
If you have plan to become a master of Crude oil, its production, and its basics, then check out the following online video courses:

I appreciate the information