Inerting refers to the process of introducing an inert gas into an enclosed space to release the gas already present, resulting in a kind of hazard. Although this process can be used to displace toxic gases, it is most commonly used to displace oxygen or to reduce the oxygen concentration in a space when the space is completely enclosed and substitution is not possible.
An inerting system reduces the combustion potential of flammable materials that are stored in a confined space. A common example of such a system is a combustible liquid-filled fuel tank that transports gasoline, diesel fuel, jet fuel, aviation fuel, or rocket propellant. Once the fuel tank is filled and during use, a space vapor barrier above the fuel known as the ullage is created. It contains evaporated fuel mixed with air containing oxygen, the important element necessary for combustion. An inerting system is used to replace the air with inert gas, such as nitrogen, argon, helium, etc. to avoid the combustion hazard.
Reason for Inerting
There are many chemical processes where inerting is required
- to make the system or process explosion-proof
- to eliminate unwanted reactions
- to keep food away from moisture
- to ensure safety during maintenance work.
Engineers often rely on inert gases and specialized inert equipment, as these goals cannot always be achieved by technology and equipment design alone. There are many situations where deactivation is the only way to meet safety standards during the production process and maintenance. In other cases, inerting is used to improve product quality.
Basic Principle of Inerting
The basic principle of inerting is to completely or partially replace air containing oxygen and flammable and/or toxic gases that often contain moisture or inert gases.
Inerting is based on the principle that a combustible (or combustible) gas can only burn (explode) when mixed with air in the correct proportions. The flammability limit of the gas determines this ratio, i.e. the flammability range. In terms of combustion technology, it can be said that the inert gas inlet dilutes the oxygen below the limiting oxygen concentration. Inerting prevents the formation of a flammable mixture that spreads by definition. Inerting introduces an inert gas to make the flammable mixture safe.
Gases Used for Inerting
The most widely used gases for inerting are Nitrogen and Carbon dioxide. Other gases such as argon and helium are applied in certain instances. Steam and exhaust gases are used in some industrial applications.
Selecting the Inert Gas
There are various criteria that influence inert gas selection. Some of these are:
Dangers of fire and explosion and/or their effects or actions
Nitrogen and carbon dioxide are not completely inert, but they are the best gases at room temperature. At high temperatures, nitrogen reacts with very few substances such as lithium, which forms lithium nitride.
6 Li + N2 → 2 Li3N
Nitrogen can react with magnesium, Silicon at temperatures between 400 ° C and 1800 ° C for nitriding, titanium, and other metals:
2 Ti + N2 → 2 TiN
The reaction with oxygen occurs only at very high temperatures. In comparison, carbon dioxide reacts with a longer list of substances containing strong bases. Amine, anhydrous ammonia, lithium, potassium, sodium, magnesium, beryllium, aluminum, chromium, manganese, titanium, uranium, and acrolein. The decomposition temperature of Carbon dioxide is 2000° C, but this is rarely a problem. However, increasing the pressure increases the solubility of this gas.
Effect on product and exhaust gas
When steam is used for inactivation, the condensate produced can sometimes be hazardous. inactivation. When shipping, readily available exhaust gases are used in the oil tank, so the fuel-to-air ratio must be carefully controlled.
Cost
Inert gases are generally too expensive to use for inertization and are not as effective as inert gases. nitrogen. However, they are often used to inactivate light metal dust. For example, Aluminium dust explosions can be prevented by using argon and that is why it is used in fire extinguishing systems for such explosions.
Specific heat capacity of inert gas
The specific heat capacity of a given inert gas determines its effectiveness. The higher the specific heat capacity, the higher the inert efficiency. Table 1 shows the corresponding values.
| Inerting Agent | Specific Heat* (Btu/(lb mole/0F) | Specific heat* (kJ/kmol/K) |
| Nitrogen | 7 | 29.308 |
| Carbon Dioxide | 8.8 | 36.844 |
| Helium | 5 | 20.934 |
| Argon | 5 | 20.934 |
| Steam | 17 | 71.176 |
The reason for this is that diluting with a high specific heat inert gas reduces the areas at risk of ignition. According to this logic, carbon dioxide is again favored over nitrogen because less inert gas is required.
Molecular structure of inert gas
The molecular structure of an inert gas directly affects its efficiency. Carbon dioxide is a triatomic molecule, nitrogen is a diatomic molecule, argon, and helium are monoatomic molecules. Polyatomic molecules can absorb more energy because they contain more free molecular vibrations. Therefore, the inert effect decreases in the following order:
CO2 → N2 → He, Ar
Figure 1 shows the effectiveness level of the inactivating agent, using the flammability of methane as an example.
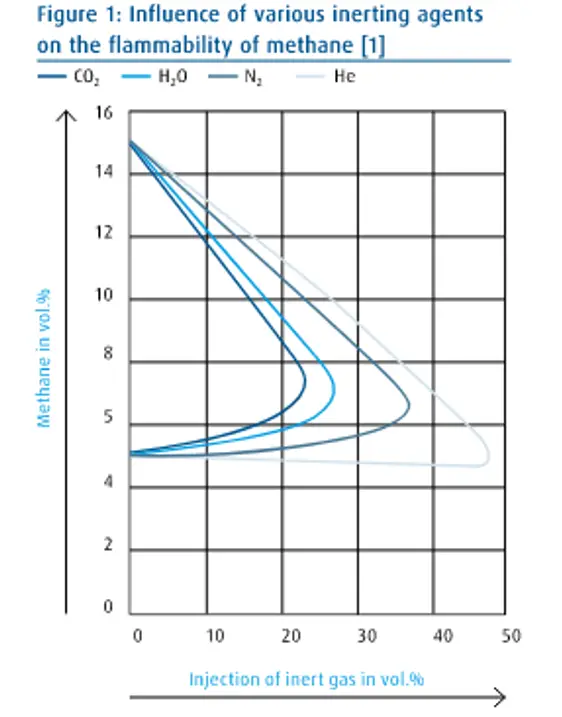
The density of inert gas
Density also plays a particularly important role. If the density of the Inactive gas is greater than the density of the replacement gas, the free space above the Inert gas injection point may not be deactivated.
Table 2 shows a comparison of the density of the selected gas to that of air.
| Types of Gas | Density in Kg/m3 at 1013 m-bar and 00C | Density relative to Air |
| Air | 1.293 | 1 |
| Nitrogen | 1.2505 | 0.967 |
| Hydrogen | 0.0899 | 0.0696 |
| Argon | 1.7837 | 1.38 |
| Oxygen | 1.429 | 1.105 |
| Carbon dioxide | 1.9767 | 1.529 |
Application of Inerting in Chemical Industries
The chemical industry uses a wide range of materials, technologies, and devices. Inactivation or inerting is especially used for:
- Reactor, Mixer
- Centrifuge and vacuum filter
- Wheat mixing plant
- Tank farms, ships
- Dryer, silo
- Gas station
- Oil line and fuel line
- Industrial service
Objectives of Inerting Activities
The predominant objectives of those inerting activities are to
- Prevent explosive atmospheres from forming in equipment along with reactors
- Ensure secure begin-up and shutdown of flora and equipment
- Avoid explosion dangers in the course of garage and delivery of flammable substances
- Protect merchandise in opposition to atmospheric oxygen whilst oxidation reactions might impair fine
- Protect in opposition to atmospheric moisture, both to keep product fine or to make sure optimum downstream processing, as an example in grinding
- Prevent fitness and protection risks in the course of the renovation of flora, equipment, and pipelines.
Different Modes of Inerting Application
Continuous Applications:
- Protection of the production process to prevent fire, explosion, and oxidation (for quality assurance)
- Inert solvent containers and transport equipment to prevent fire and explosion
Intermittent applications:
- Purge pipeline
- Tank purging
- Deactivation of the filter system
- Inactivation of silos
- Deactivation of grinding equipment
- Smoke evolution in the mine
Types of Inerting
Depending on the application, inerting can be classified into two groups as mentioned below:
Partial inerting:
The oxygen concentration is reduced to a level low enough that the mixture will no longer explode. In the case of partial inertia, the goal is to reduce the oxygen concentration in the mixture to a level where it will no longer explode.
Complete inerting:
Complete inertia involves increasing the ratio of inert gas to a combustible material to a level at which the addition of any amount of air cannot produce an explosive mixture.

You state: “The higher the specific heat capacity, the higher the inert efficiency”.
Steam has roughly twice the heat capacity of CO2 so from your criterion the inert efficiency of steam must be roughly twice that of carbon dioxide.
But Fig 1 shows the inert efficiency of steam is significantly less than CO2. So what do you mean “The higher the specific heat capacity, the higher the inert efficiency”?
Hello, my name is Jack chen and I am from a university in China. Seeing your article, I really appreciate the professional work you have done. I am doing water electrolysis to produce hydrogen, and I plan to have nitrogen for inerting, which can improve safety. So my question is if hydrogen, oxygen, and nitrogen are mixed, what is the range of gas-to-volume ratios of oxygen that is safe? If you can reply to my email, we can discuss how we can work together. I need to fix this.