Stainless steel is renowned for its exceptional resistance to corrosion, making it a popular choice in various industries, including construction, manufacturing, and food processing. However, there is often confusion surrounding the potential for stainless steel to rust or corrode.
“Does Stainless Steel Rust?” This question sometimes arises in every material engineer’s mind whenever they learn about stainless steel. It is well known that stainless steel is specifically prepared to provide it with in-build corrosion resistance by adding a sufficient amount of chromium. So, it is believed that stainless steel is rust-resistant. In this article, we will find out the answer to the most popular question “Does stainless steel rust or corrode?”
Rust Resistance Mechanism of Stainless Steel
The mechanism of stainless steel’s corrosion resistance is as mentioned below:
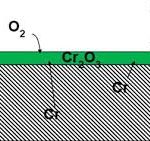
Stainless steel contains more than 10.5% chromium. Surrounding oxygen of air reacts with this chromium and forms a thin stable chromium oxide layer that musks the steel surface. This chromium oxide layer works as a passive barrier film that does not allow the air and water to access the underlying metal and thus protects the surface from rusting. This is the reason that stainless steel is rust-resistant and this alloy is preferred for countless manufacturing applications.
Certain specific alloying additions increase the corrosion resistance properties of stainless steel. For example, molybdenum. Because of the presence of molybdenum grade 316 stainless steel is more corrosion resistant as compared to grade 304 stainless steel.
Does Stainless Steel Rust?
Yes, Stainless steel does rust or corrode in certain situations. There are various factors that can corrode or develop rust in stainless steel. Some of the stainless steel corrosion mechanism are mentioned below:

Pitting Corrosion in Stainless Steel:
Certain types of stainless steel can rust when exposed to strong chlorides. For example, the corrosive nature of chlorine in seawater or pool water can cause the stainless steel to rust or corrode. This is known as pitting corrosion in stainless steel. This type of rusting of stainless steel can be prevented by:
- Using the superior grade of stainless steel (for example SS316 is resistant to chlorides).
- Applying a specialized coating to prevent direct contact with the chloride environment.
- Proper cleaning of the surface.
Galvanic Corrosion due to Dissimilar Stainless Steel Alloys:
Sometimes, two different grades of dissimilar alloys are welded together to prepare some custom products. This creates a galvanic current flow between them and the anode starts to corrode. This phenomenon is known as galvanic corrosion. The weld filler can also cause galvanic corrosion. This type of stainless steel corrosion or rusting can be prevented by:
- avoiding joining dissimilar metals.
- adding a coating to seal the metal off for electron flow.
General Corrosion of Stainless Steel:
When the stainless-steel surface comes into contact with an acid-based material, general corrosion may occur in stainless steel. It creates uniform metal loss from the entire surface. Stainless steel components having a pH value of lower than 1 have a greater tendency to be attacked by general or uniform corrosion.
Transplanting of Plain Iron or Steel onto Stainless Steel:
Sometimes residue from iron or steel parts is transferred to stainless steel components. For example, while cleaning stainless steel utensils or cookware using steel wool or wire brush. If these residue particles are exposed to humid air or moisture, rusting starts. This type of stainless steel rust can be prevented by thorough cleaning of the surfaces to avoid the deposition of iron or normal steel over the SS surface.
Applying Temperature Extremes to Stainless Steel:
When stainless steel is exposed to extreme temperatures (750 to 15500F) the rust-resistance capability reduces. For example, during welding (or heat treatment) of stainless steel, high heating and cooling generate a process called sensitization where carbon and chromium bond together to form carbides. Due to these, the amount of chromium reduces in stainless steel making it prone to rusting. To prevent stainless steel rusting due to temperature extremes the product must be operated in its operating temperature range.
Chemical Corrosion in Polluted Air
In prolonged exposure to polluted air (containing larger amounts of sulfides, oxides, and hydrogen oxide), in the case of condensed water, sulfuric acid, nitric acid, and acetic acid liquid spots are formed on the stainless steel surface causing chemical corrosion.
Removing Rust from Stainless Steel Surfaces
There are various methods by which the rust from the stainless steel surface can effectively be removed. For example, By dissolving the rust or iron oxide using weak acids like phosphoric acid or acetic acids can help substantially to get rid of the rust. These acids do not react with the parent metal and simply dissolved the rust to provide a cleaner surface.
Preventing Stainless Steel from Rusting
The best effective way to prevent stainless steel from rusting is to powder coat the stainless steel surface by depositing a dry-colored powder using electrostatic charges. Upon heating the surface, the powder coating cures to a hard finish, which results in a full coating of the bare stainless steel in an attractive and protective layer. Also, stainless steel passivation will reduce the rust or corrosion in stainless steel material.
What can cause stainless steel to rust?
In general, stainless steel with its chromium oxide layer works as corrosion-resistant material. However, in certain environments when that chromium oxide layer is damaged, stainless steel can rust. Certain chlorides, cleaners, high humidity, mechanical abrasions, or high salinity environments can damage the protective layer of stainless steel, making it prone to rust formation.
Does stainless steel rust in water?
No, in normal water, stainless steel does not rust. You must have experienced the same with your stainless steel kitchen utensils. They are washed every day with water but do not produce rusting.
Does stainless steel rust over time?
If stainless steel is maintained properly and kept away from harsh environments it will not rust.
What does saltwater do to stainless steel?
With constant exposure to saltwater, stainless steel can rust over time.
Factors Influencing Stainless Steel Corrosion
Although stainless steel is highly resistant to corrosion, it is understood that certain factors can impact its corrosion resistance:
- Environment: The specific environment in which stainless steel is exposed plays a crucial role in its corrosion resistance. Factors such as temperature, humidity, exposure to chemicals, and the presence of chlorides or acids can influence the corrosion behavior of stainless steel. Severe environments with high chloride concentrations, such as coastal areas, can pose challenges to stainless steel’s corrosion resistance.
- Surface Finish and Cleaning: The surface finish of stainless steel affects its corrosion resistance. Smooth, polished surfaces are more resistant to corrosion than rough or pitted surfaces. Regular cleaning and removal of contaminants, such as dirt, oils, and debris, help maintain the protective passive film on the stainless steel surface, ensuring optimal corrosion resistance.
- Alloy Composition: The composition of stainless steel, including the percentage of chromium, nickel, and other alloying elements, influences its corrosion resistance. Higher chromium and nickel content generally improve corrosion resistance. The selection of the appropriate stainless steel grade for a specific environment is crucial to ensure optimal performance.
Comparative Advantage of Stainless Steel
When compared to other metals, stainless steel offers distinct advantages in terms of corrosion resistance:
- Iron and Mild Steel: Unlike iron and mild steel, which are highly susceptible to rusting, stainless steel forms a protective chromium oxide layer that prevents rust and corrosion. This makes stainless steel a more durable and long-lasting material.
- Aluminum: While aluminum is known for its corrosion resistance, stainless steel often surpasses it in harsh environments, particularly when exposed to chlorides or acidic conditions.
- Galvanized Steel: Galvanized steel, which is coated with a layer of zinc, offers corrosion resistance but may be less durable in certain aggressive environments compared to stainless steel.
Conclusion
Stainless steel’s resistance to corrosion sets it apart from many other metals. Its ability to form a protective chromium oxide layer enables it to withstand a wide range of corrosive environments. However, the corrosion resistance of stainless steel can be influenced by factors such as the environment, surface finish, cleaning practices, and alloy composition. By understanding these factors and selecting the appropriate stainless steel grade for specific applications, engineers and manufacturers can harness the full potential of stainless steel and ensure its long-term durability and performance.






Very good.
I discovered a small patch of brownish stain (rust) on the underside of my stainless steel watch. I was quite shock cause I didn`t expect it to rust. I then try to scape it off using my fingernail and wipe it off with a microfibre cloth. It was slowly removed and back to pristine state.
My question is doesnt rusting form when corrosion take place but why It can be removed and the surface appear shiny again. Then how do the rust even form in the first place.