Gas hydrates are ice-like crystalline minerals normally formed from methane and water. When Low molecular weight gases solidify at low temperatures and moderate pressure conditions, gas hydrates are formed. In marine sediments and permafrost, gas hydrates can occur naturally. It is believed that gas hydrates are present on other planets, too.
As exploration and development activities have moved into deeper water, gas hydrates are an increasingly important concern. This presentation gives an overview of gas hydrates including what they are, why there is so much interest in them, and ends with what areas are of interest to MMS, both in the short term and long term.
What are Gas Hydrates?
Gas hydrates are crystalline or ice-like structure that forms a cage around a molecule of gas, as you can see from Fig. 1. The water molecules are bonded to form the structure and, in this case, a methane molecule is trapped inside. The gas that is trapped can be methane, carbon dioxide, hydrogen sulfide, and some smaller hydrocarbon molecules like ethane, butane, and propane.
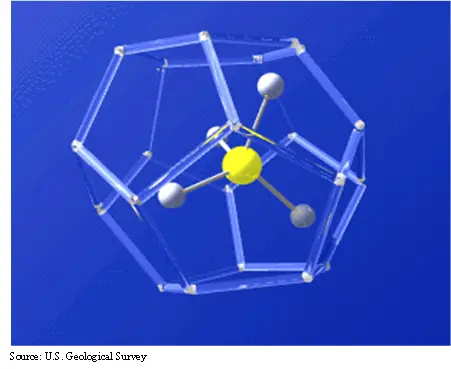
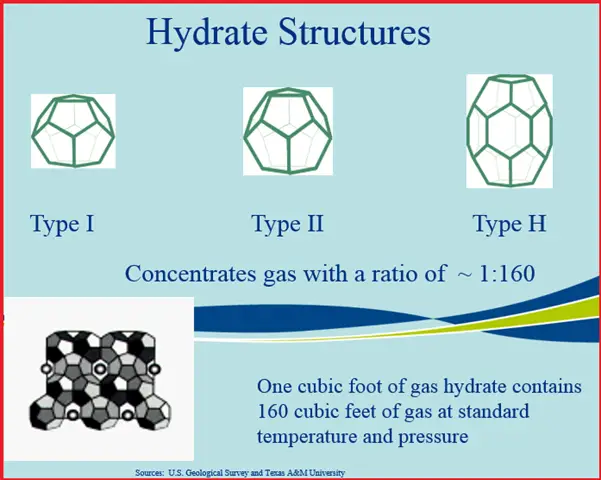
The cage can take several forms as seen in Fig. 2. All three have been observed in nature, but they can also be synthesized in the laboratory. The interest in methane hydrates as a potential resource result from the trapping capability of the hydrate structure. Essentially, hydrates concentrated gas by a ratio of 1:160. What this means, is that in one cubic foot of hydrate, about 160 cubic feet of gas is trapped. This would be 160 cubic feet at standard temperature and pressure.
Elements for Gas Hydrate Formation
The formation of gas hydrates needs the following five Ingredients
- Water
- Gas – CH4, CO2, C2H6, H2S, etc.
- Pressure
- Temperature
- Nucleation Site
How are gas hydrates formed?
For gas hydrates to form, several ingredients are necessary. Of course, you need water to form the cage and gas to fill the cage. The reason that hydrates are of interest in deep water is that they also need high pressures and low temperatures. In addition, a nucleation site is required. The nucleation site would be a surface such as a grain of clay or a pipeline or a piece of the platform. This is why you don’t find hydrates floating around in seawater.

Fig. 3 shows some examples of hydrates that have been recovered. In the top picture, you can see the white banding, which is the hydrate. The lower left picture is a nodule of hydrate and on the right is a hydrate recovered from the Gulf of Mexico, which is coated with oil. As you can see, hydrates from within the sediments and you would not expect to find huge blocks of hydrate in the sediments, there isn’t room for them to form.
Disadvantages of Gas Hydrates
There are several reasons for the interest in gas hydrates. The safety issues include the plugging of flowlines and geohazards. Methane hydrate is considered a potential resource. And of environmental concern, sensitive biological communities exist on outcrops. Hydrates may also contribute to global warming.
Safety Issues with Gas Hydrates:
- Gas Hydrates plug flowlines
- Gas Hydrates can be geohazards
Resource:
- Methane Hydrates are a source of natural gas
Environmental:
- Sensitive Communities use hydrates as food
- Methane Hydrates can contribute to global warming
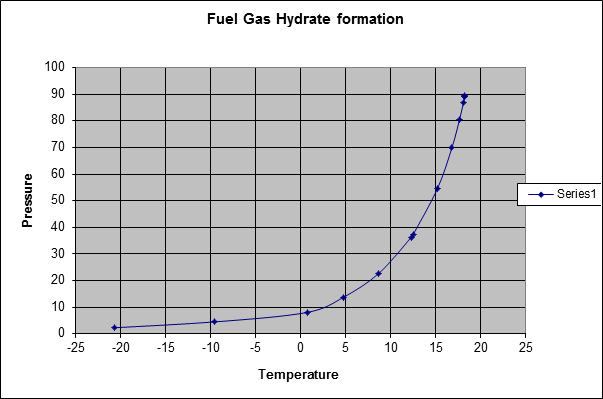
Gas Hydrate curve
Fig. 4 shows a typical gas hydrate curve
Chemical Injection
- Scale Inhibitor- To reduce the scale formation due to the precipitation of mineral compounds present in water
- Gas Hydrate- Inorganic salts/ crystals containing water molecules
- Demulsifier– Emulsion breaker (Use to separate emulsion e.g. water in oil)
- Corrosion Inhibitor–To reduce the rate of corrosion ( Anodic Inhibitor / Cathodic Inhibitor / Oxygen scavenger)
- Microbicides – Antiseptics used to counter microbial corrosion
Scale Inhibitor:
Scale is a hard crystalline deposit resulting from the precipitation of mineral compounds present in water.
Oilfield scales typically consist of one or more types of inorganic deposits along with other debris organic precipitates, sand, corrosion products, etc.
Carbonate and Sulfate scales – Add to pressure drop
Cause of scale deposits
- formation damage (near well bore)
- blockages in perforations, gravel packs, or screens
- restrict/block flow lines
- safety valve & choke failure
- pump wear
- corrosion underneath deposits
- some scales are radioactive
- Suspended particles
- plug formation & filtration equipment
- reduce oil/water separator efficiency
Scaling (Fig.-5):

