What is ORP?
ORP stands for oxidation-reduction potential, which is a measure, in milli-volts, of the tendency of a chemical substance to oxidize OR reduce another chemical substance.
- OXIDATION: Oxidation is the loss of electrons by an atom, molecule, or ion.
- REDUCTION: Reduction is the net gain of electrons by an atom, molecule, or ion.
- OXIDATION: Fe = Fe+2 + 2 e- (Half- Reaction)
- REDUCTION: Cl2 + 2 e- = 2 Cl- (Half-Reaction)
- OVERALL REACTION: Fe + Cl2 => FeCl2
In the reaction above, iron (Fe) reduces chlorine (Cl2) and is called a reductant or reducing agent. Conversely, chlorine (Cl2) oxidizes iron (Fe) and is called an oxidant or oxidizing agent:
ORP Measurement
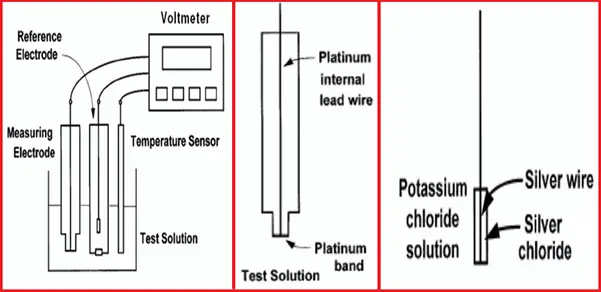
- The cell consists of a measuring and reference electrode.
- The voltage between the electrodes is the ORP of the test solution.
- Although the electrodes are shown separately, in most processes ORP sensors, the electrodes, and the temperature element are combined into a single sensor.
- Because ORP depends on temperature, the temperature at which the measurement is made must be reported.
Measuring Electrode
An ORP electrode (Fig. 1) is a piece of noble metal, usually platinum,
but sometimes gold, attached to the end of a glass tube. The potential of the electrode is controlled by the ratio of oxidized to reduced substances in the sample. pH and other constituents in the sample may also affect ORP.
The Reference Electrode
The reference electrode used for ORP measurements is typically the same silver-silver chloride electrode used with pH measurements. In contrast with pH measurements, some offset in the reference is tolerable in ORP since, as will be seen, the mV changes measured in most ORP applications are large.
In certain specific applications (for example, bleach production), an ORP sensor may use a silver billet as a reference, or even a pH electrode.
The electrode behavior is described by the Nernst equation:
Em = E o – (RT/nF) ln {[ox] / [red]}
Where,
- Em is the potential from the ORP electrode,
- Eo is related to the potential of the reference electrode,
- R is the Gas Law constant,
- F is Faraday’s constant,
- T is the temperature in Kelvin,
- n is the number of electrons,
- [ox] is the oxidant concentration in moles/L, and
- [red] is the reductant concentration in moles/L.
Why is ORP Measured?
Applications of the ORP measurement are not nearly as widespread as those of pH, but several reasonably standard applications exist.
- Bleaching operations
- Manufacture of bleach
- Oxidation of cyanide wastes
- Reduction of chromate wastes
Temperature Effects on ORP Measurement
The temperature has 2 distinct effects on ORP measurements:
- Electrodes will have a different output potential at different temperatures for a given ratio of ionic activity.
- Ionic activity is affected, arising from temperature effects on dissociation, activity coefficients, and interactions between ions in solution.
In general, however, ORP measurements are not compensated for temperature effects due to the fact that:
- The isopotential point (the point of thermal independence) of ORP systems is specific only for that particular redox reaction (i.e. there is no “standard” point for all ORP reactions).
- The chemistry of the redox reaction can be quite complex, especially if several ionic species involving varying numbers of electrons transferred (thus varying values for n within one equation) contribute to the reaction/oxidation-reduction potential.
- Most ORP measurements are done at constant temperatures, such as in process measurement and control.
How to Approach ORP Applications?
Regardless of the nature of the application under consideration, the steps to follow are the same:
- Look up the half-reaction(s) of the redox couple(s) involved in the application in a handbook of chemistry or other references.
- Not all of the chemical substances involved in the half-reaction and their concentration range in the application under consideration.
- Substituting their minimum and maximum concentrations into the Nernst equation can provide the contribution of each substance to the overall ORP.
- If an oxidation-reduction reaction is being monitored or controlled, the equivalence point can be calculated over the pH range of the process.
ORP -Cyanide Treatment in Metal Industry
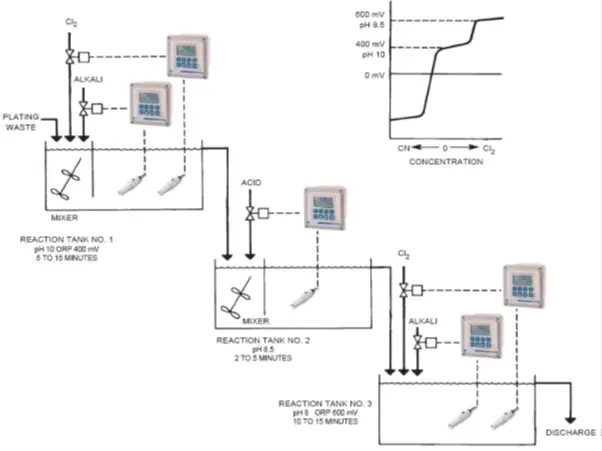
PROCESS: One of the most effective means to treat these waste materials is to destroy the cyanide with chlorine.
This process is accomplished in two stages:
- Cyanide is oxidized to cyanate, using pH and ORP control.
- Cyanate is oxidized to nitrogen and carbon dioxide, harmless gases, that can be safely discharged into the environment. This stage also uses pH and ORP control.
OXIDATION OF CYANIDE TO CYANATE:
In the first reaction tank, the pH of the waste is measured, and caustic (NaOH at 50% strength) is automatically injected to raise the pH to 10 or higher.
The oxidation-reduction potential (ORP) of the waste is measured, and chlorine gas (Cl2) is automatically injected to raise the ORP to 400 mV or higher.
The following reaction occurs, taking from 5 to 10 minutes:
NaCN + Cl2 + 2NaOH NaCNO + 2NaCl + H2O
DESTRUCTION OF CYANATE TO NITROGEN AND CARBON DIOXIDE:
In the second reaction tank, the pH of the waste is measured, and acid is injected to lower the pH to 7-8.
This process takes 2 to 5 minutes. In the third reaction tank, the ORP of the waste is measured and chlorine gas (Cl2) is automatically injected to raise the ORP to 600 mV or higher (Meanwhile the pH controller maintains the set-point at 7-8, correcting for any acidity created by the addition of the chlorine gas).
The following reaction occurs, taking 10 to 15 minutes:
2NaCNO + 3Cl2 + 4NaOH 6NaCl + 2CO2 + N2 + 2H2O
The cyanide is eventually converted to harmless materials by the above reaction and the waste can be discharged.






Dear Anup Kumar Dey,
I would like to know any Standard is there for Pipe Support. I have faced a problem form my Client who has asked to us to use Statdpro even for the pipes having working pr. of 7 kg/sqcm and Diameters most of them 4″ and only few 14,” supported on simple steel sections and height of the supports are less than 2500mm. We have successfully completed the project. But I think it was a waste of time , it was just done to satisfy ego of Client.