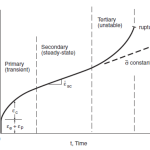
Refractory metals are a unique category of metallic materials known for their exceptional resistance to heat and wear, making them invaluable in various high-temperature applications. “Refractory Metals” in engineering refers to a group of materials having an extremely high melting point. They can retain their shape and usefulness at high temperatures and have a very high melting point. In general, refractory metals meet the following two basic criteria:
- Their melting point is above 4000° F (2200° C) and
- Their creep resistance is above 2700° F (1500° C)
In this article, we will explore the properties, types, and common applications of refractory metals.
What Are Refractory Metals?
Refractory metals are defined as metals that have exceptionally high melting points and retain their strength and stability at elevated temperatures. These metals are crucial in applications that require materials to withstand extreme conditions without deforming or losing integrity.
The study of refractory metals began in the early 20th century, primarily focused on tungsten and molybdenum, which were used in electrical contacts and filaments. Over time, the unique properties of these metals led to their adoption in a wide range of industries, particularly as technology advanced and the demand for materials that could endure harsh environments increased.
Refractory metals are a broad class of metals having excellent heat resistance, extreme wear resistance, and very high hardness at room temperature. There are 5 metals that are undisputedly considered refractory metals. They are:
- Tungsten (W): Melting Point 3420°C, BCC
- Rhenium (Re): Melting Point 3185°C, HCP
- Tantalum (Ta): Melting Point 3017°C, BCC
- Molybdenum (Mo): Melting Point 2623°C, BCC
- Niobium (Nb): Melting Point 2477°C, BCC
There are some other metals that display similar properties and are broadly referred to as refractory metals. they are:
- Osmium (Os): Melting Point 3027°C, HCP
- Iridium (Ir): Melting Point 2447°C, FCC
- Ruthenium (Ru): Melting Point 2250°C, HCP
- Hafnium (Hf): Melting Point 2227°C, HCP
- Technetium (Tc): Melting Point 2200°C, HCP (Radioactive)
- Rhodium (Rh): Melting Point 1963°C, FCC
- Vanadium (V): Melting Point 1902°C, BCC
- Chromium (Cr): Melting Point 1857°C, BCC
- Zirconium (Zr): Melting Point 1852°C, HCP
- Titanium (Ti): Melting Point 1670°C, HCP
Properties of Refractory Metals
All refractory metals have some unique and desirable properties as listed below:
Physical Properties of Refractory Metals
Refractory metals are distinct for the following key physical properties:
- Very high boiling point.
- High creep resistance.
- High density
- Heat and electrical conductivity.
- All refractory metals have a close-packed or nearly close-packed crystal structure.
Strength and Hardness
Refractory metals exhibit excellent mechanical properties, including high strength and hardness, even at elevated temperatures. This makes them suitable for use in high-stress applications, such as turbine components and cutting tools.
Oxidation Resistance
Many refractory metals possess good oxidation resistance, particularly at high temperatures. This characteristic is crucial for applications in oxidizing environments, such as aerospace and nuclear applications, where prolonged exposure to oxygen can lead to material degradation.
Thermal Conductivity
Refractory metals typically have high thermal conductivity, which allows for efficient heat dissipation. This property is particularly valuable in applications involving heat sinks and thermal barriers.
Chemical and Mechanical Properties of Refractory Metals
Some of the chemical and mechanical properties of refractory metals are:
- They oxidize easily and create a stable oxide outer layer which gives them high corrosion resistance.
- Thermal shock resistance.
- Refractory metals are usually stable against acids.
- Refractory metals have high strength and extreme hardness.
- Outstanding Abrasion and Wear Resistance
- Low diffusion rates
Processing of Refractory Metals
The processing of refractory metals presents unique challenges due to their hardness and melting points. Below are some common techniques employed to fabricate these metals.
Powder Metallurgy
Powder metallurgy involves the use of metal powders to create components through compaction and sintering. This method is particularly effective for refractory metals, as it allows for precise control over the microstructure and properties of the final product.
Arc Melting
Arc melting is a technique used to melt and refine refractory metals by creating an electric arc between two electrodes. This method is useful for producing high-purity alloys and is often employed in research and small-scale production.
Chemical Vapor Deposition
Chemical vapor deposition (CVD) is a process used to produce thin films of refractory metals on substrates. CVD is valuable for applications requiring precise coatings, such as semiconductor manufacturing.
Additive Manufacturing
Additive manufacturing, or 3D printing, is becoming increasingly important for refractory metals. This technique allows for the production of complex geometries and reduces material waste, making it an attractive option for many industries.
Applications of Refractory Metals
The most commonly used refractory metals are tantalum, niobium, molybdenum, and tungsten. They are combined with other materials to create an extensive range of refractory compounds. the major applications of refractory metals are listed below:
Electronics and Semiconductors Industry: Due to strong electrical conductivity, refractory metals are used to produce durable electrical components by alloying with copper, gold, or silver. They are widely used for producing cases, anodes, and cathodes.
Industrial Parts: Refractory metals are suitable for applications involving extreme thermal and mechanical stresses. Hence, they are widely used in glass melting electrodes, furnace boats, sintering trays, rods, sheets, crucibles, shields, tubes, and nozzles.
Medical Industry: Various medical devices are produced using refractory metals. For example, Tantalum is used in dental devices, surgical clips, bone grafts, Plates for cranioplasties, etc. Molybdenum is used in medical scanning tools, Tungsten is used for radiation shields, and tantalum and niobium are used in MRI scanners.
Nuclear Applications: Niobium, molybdenum, and tungsten are widely used in the nuclear industry. Tungsten is utilized in radiation shielding, whereas niobium zirconium alloy is used for nuclear reactor structural components.
Aerospace and Defence Applications: Due to very high thermal and mechanical durability, refractory metals find wide applications in the defense and aerospace industry. Molybdenum, tungsten, niobium, and tantalum have got a range of aerospace applications in forging dies, thrusters, shields, and balance weights.
Chemical Industry: Tantalum is widely used in heat exchangers, reaction vessels, and spargers to reject the corrosive effect of various acids and chemicals.
Superconductors: Tantalum and niobium are widely used in low-temperature superconductor applications, mass spectrometry, NRM, particle accelerators, MRI medical imaging, and various analytical and experimental equipment.
Powder Metallurgy: Refractory metals are highly popular in powder metallurgy. They are processed in specific powder sizes and forms and then blended to get the required mixture of properties.
Superalloys: Rhenium is an additive for producing superalloys. The creep strength of superalloys increases by a great extent with the addition of rhenium in conjunction with iron, cobalt, nickel, tungsten, and molybdenum which makes these alloys suitable for gas turbine and jet engine part manufacturing.
Tungsten is widely used in lighting applications, industrial ovens, and various types of probes.
In conclusion, Refractory metals are a vital component of modern technology, offering unparalleled properties that make them suitable for a wide range of high-temperature applications. From aerospace to electronics, their unique characteristics enable innovations that drive progress across various industries. Despite the challenges associated with their use, ongoing research and development promise to unlock new potential for these remarkable materials, ensuring their relevance in the future. As we move towards a more advanced and sustainable world, the importance of refractory metals will only continue to grow.