This article introduces the main existing technologies to carry out a conversion line of a waste stream into an energy-usable gas mixture (syngas). The syngas also serves as an intermediate point in the synthesis processes of various high-added-value products.
Introduction
Faced with the problems of climate change and global warming, research and development have focused on the use of biomass as an alternative to fossil fuels, among other energy sources. The wide availability of waste has been widely recognized for its potential to provide greater amounts of useful energy with less environmental impact than fossil fuels.
Waste can be converted into a commercial product through biological or thermochemical processes. The biological conversion of biomass continues to face challenges related to low efficiency and cost-effectiveness. In the case of thermochemical processes, combustion, pyrolysis, and gasification are the three main conversion methods.
Biomass is traditionally burned to provide heat and electricity in industrial processes. The net efficiency for electricity generation from its combustion is usually very low, not exceeding 50%. Biomass combustion is usually limited to 10% of the total feedstock due to, among other things, carbon lock-in in existing feed systems. Pyrolysis, on the other hand, converts biomass in the absence of oxygen. Limited uses and the difficulty of downstream processing have restricted the wide application of biomass pyrolysis technology.
Finally, gasification converts biomass by partial oxidation into a gaseous mixture, with small amounts of carbon and condensable compounds. It is considered one of the most efficient ways to convert the energy stored in biomass and is becoming one of the best alternatives for the reuse of solid waste.
A process that is currently being developed in several parts of the world is the gasification of municipal solid waste (MSW), in which syngas is produced. After gasification, the syngas is treated to remove the main contaminants. Once the gas meets the necessary requirements, it can be used as an intermediate product for different processes, such as the production of SAF (sustainable aviation fuel) by Fischer Tropsch synthesis, or to produce DME and methanol, among other products of great interest on the world scene.
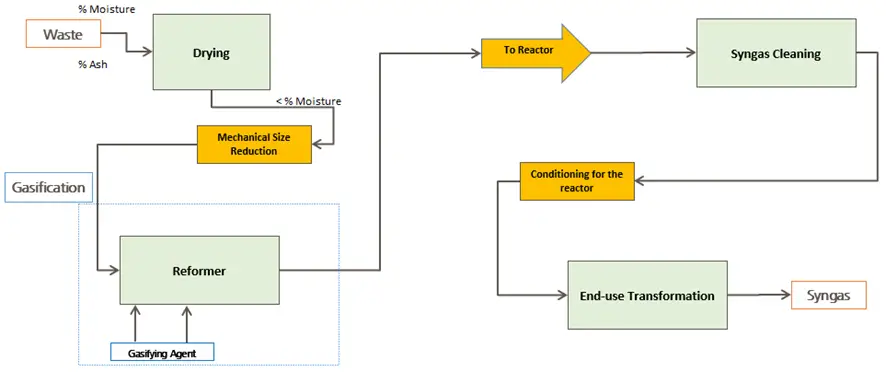
What is Gasification?
Gasification is a partial thermal oxidation resulting in a high proportion of gaseous products (CO2, H2O, CO, H2, and gaseous hydrocarbons), small amounts of carbon (solid product), ash, and condensable compounds (tars and oils).
Air, steam, oxygen, or a mixture of these is supplied to the reaction as the oxidizing agent. The gas produced can be standardized in quality and is easier and more versatile to use than the original biomass (e.g., it can be used to power gas engines and turbines or as a chemical feedstock to produce liquid fuels).
Gasification adds value to low-value feedstocks by converting them into marketable fuels and products.
Stages of Gasification
In conventional biomass treatment plants, energy is obtained through incineration or gasification.
The biomass gasification process allows energy to be obtained in the form of heat or electricity, using the syngas (synthesis gas) to drive the shaft of a turbine, or burning it as fuel to drive an engine. This process typically uses coal as a feedstock.
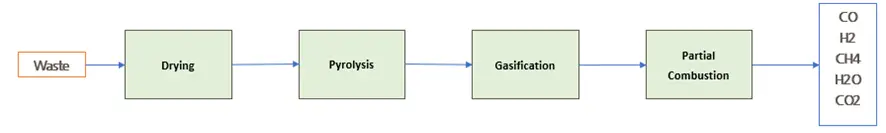
The chemistry of biomass gasification is quite complex. In general terms, the gasification process consists of the following stages (drying, pyrolysis, gasification, partial combustion):
Gasification Reactor Design
Gasification reactor designs have been investigated for more than a century, resulting in the availability of several small and large-scale designs. They can be classified in several ways, although we will focus on classification by design.
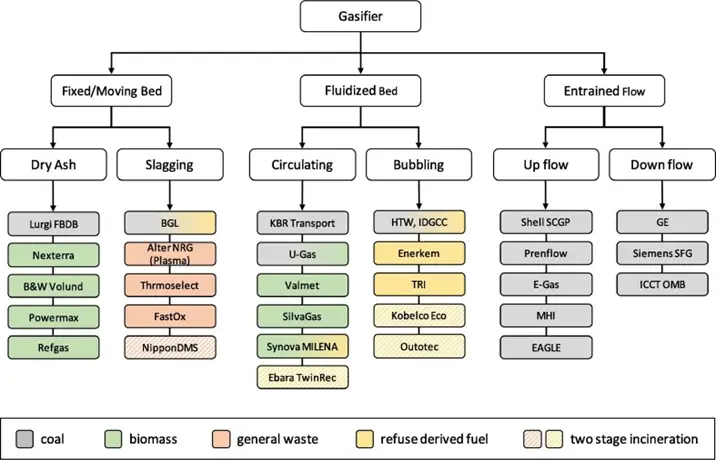
Depending on the configuration, gasifiers are classified into three main types: fixed-bed, fluidized-bed, and entrained-flow.
These gasifiers can be divided into the categories shown in the figure. Fixed-bed gasifiers are ideal for small-scale biomass feedstocks.
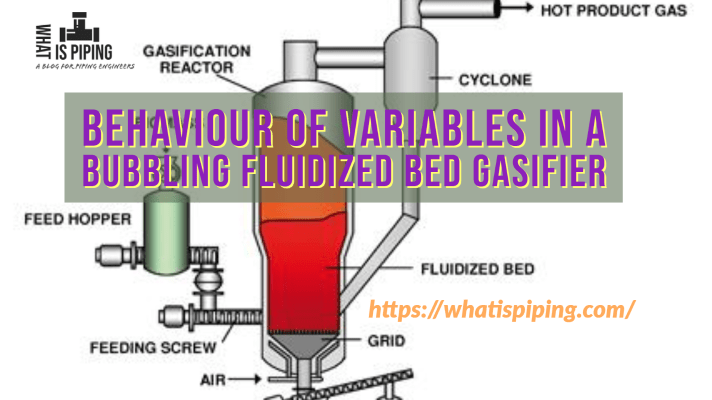
Fluidized-bed gasifiers can be used to process biomass and refuse-derived fuel (RDF) from pre-treated waste feedstocks, which must meet size, composition, and moisture content specifications.
Entrained flow gasifiers are commonly used for coal because they can be fed in direct gasification mode, which makes feeding the solid fuel at high pressures economical. Short residence time, high temperatures, high pressures, and large capacities are the characteristics of these gasifiers.
Challenges and Opportunities to Improve Gasification
The main problem arises in the heterogeneous nature of the reactor feed stream, the limited experience under commercial conditions, and the quality of the syngas obtained. Some of the problems related to waste heterogeneity are overcome by pre-treatment of the waste at the inlet of the gasifier. However, some energy is required and must be accounted for to make a proper balance.
The raw syngas may contain the following contaminants: tars, sulfur, nitrogen, and chlorine-containing gases (NH3, HCl, HCN, H2S, COS), fly ash, and particles containing K, Na, and traces of other elements that may influence catalyst performance.
Syngas or Synthesis Gas
Synthesis gas or “syngas” is a mixture composed of carbon monoxide, carbon dioxide, hydrogen, and methane. It is produced by the gasification of a carbon-containing fuel to form a gaseous product that has a certain calorific value. Examples of synthesis gas production include the gasification of carbon-rich compounds, gasification of various wastes, and steam reforming of coke.
It is a gas that is used as an intermediate product to synthesize other substances, which is why it is called synthesis gas. It is also an intermediate in the creation of synthetic oil for use as a lubricant or fuel.
Main Contaminants in Synthesis Gas
Synthesis gas contaminants are composed of tars, nitrogen-based compounds (NH3, HCN, etc.), sulfur-based compounds (H2S, COS, etc.), hydrogen halides (HCl, HF, etc.), and trace metals (Na, K, etc.).
The presence of these contaminants in the synthesis gas poses several technical and operational problems ranging from corrosion (H2S) and fouling of equipment (tar), deactivation of catalysts (tar, H2S, NH3, HCl, and trace metals), or environmental pollution (NH3).
Most downstream applications of syngas have very stringent requirements in terms of composition, so they have different levels of cleanliness requirements depending on their application. Therefore, contaminant levels must be reduced by cleaning the gas to meet the requirements of downstream applications.
Cleaning of Syngas
Cleaning the synthesis gas is an essential step prior to its further use. Cold gas cleaning is considered the most widely used cleaning method due to its proven reliability and high efficiency in removing contaminants.
The main characteristic of this method is that it is carried out at a low temperature, usually at room temperature or below.
In terms of cost, this approach is more suitable for large-scale applications due to the need to treat solvent effluents generated during gas cleaning. Since gasification is carried out at about 800°C, the main disadvantage of this method is the efficiency penalty paid due to stream cooling, in addition to the secondary cost of treating or removing contaminant streams.
Cold gas cleaning uses either dry or wet processes. Wet cold gas cleaning processes employ scrubbing towers, impingement scrubbers, Venturi, and electrostatic precipitators or cyclones. These units remove contaminants by absorption, adsorption, filtration, or a combination of these.
Wet gas scrubbing processes are the most used because they allow the removal of more contaminants.
For example, ammonium (NH3), hydrochloric acid (HCl), and hydrogen sulfide (H2S) are readily soluble in water. Therefore, scrubbers, scrubbers, and cyclones that use water as a solvent will remove all these contaminants, although with different removal efficiencies depending on their solubility in water.
Uses and Market of Syngas
MSW is a raw material that is not in short supply, widely available and is not susceptible to geopolitical risks, risks in commodity price fluctuations, or risks of natural disasters. As a waste product, it has no real competitive uses.
On the other hand, it is important to note the role of synthesis gas in the market. Throughout the chemical industry, synthesis gas is an intermediate product.
The ammonia industry dominates the world market for synthesis gas (mainly from fossil fuels such as coal, natural gas, and oil). Hydrogen production for use in refineries and methanol processing are other important applications.
Conclusions
Syngas is defined as a gas, rich in H2 and CO, the main fuel components. Its main properties are flammability limit and laminar flame speed.
Syngas is produced from biomass/coal gasification or natural gas reforming, and the yield is measured by the mass of product produced per mass of feedstock.
The Fischer-Tropsch technique is one of the commercially available methods for making clean synthetic fuel from syngas.
On the other hand, products such as methanol, dimethyl ether, ammonia, etc. can also be produced from syngas.
You can continue reading about gasification in this article.