Corrosion can be defined as the deterioration of materials under the influence of an environment. Without exception, the corrosion of metals and alloys (a majority of materials used in industry) in aqueous environments (the most often encountered environment) is an electrochemical reaction.
Factors affecting Corrosion
Corrosion behavior depends on the following factors:
- the fluid
- temperature
- flow velocity
- the atmosphere
- concentration
- dissolved oxygen
- phase
- contaminants in the flow stream
- pH
- mechanical properties of materials like hardness, the extent of cold work, grain structure, etc
- process conditions
- weld properties like heat treatment, hardness, residual stresses, inclusions, heat-affected zone, etc.
- Coating and lining conditions
- geometry like local turbulence, crevices, etc.
Types of Corrosion / Corrosion types
Depending on various corrosion mechanisms, popular corrosion types are
- Electro-Chemical Corrosion Types
- General wall thinning
- Local wall thinning
- Pitting
- Crevice
- Galvanic
- Flow Assisted Corrosion types
- Erosion
- Erosion-corrosion
- Environmental Cracking
- Inter-granular
- Trans-granular
- Stress Corrosion cracking or SCC
- Hydrogen Induced Cracking
- Fatigue
- Mechanical fatigue cracking
- corrosion fatigue
- High-temperature corrosion
- Microbiological corrosion
Fig. 1 shows the normal types of Corrosion that are prevalent in the Oil and Gas Industry.
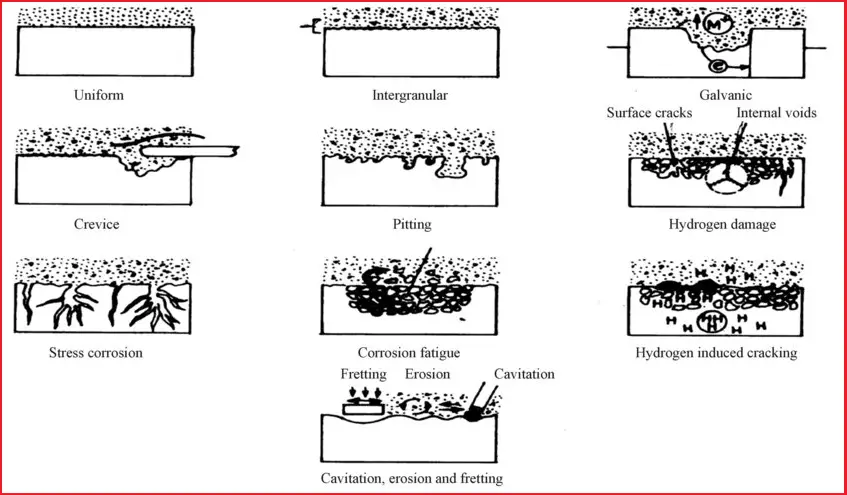
Uniform Corrosion
Though uniform corrosion is an idealized form of corrosion and because of less damage than the other forms of corrosion, it is more appropriate to understand this form of corrosion. This leads to uniform thinning of the structures.
Units of Corrosion | Corrosion Measurement
The corrosion attack is measured in terms of penetration. Corrosion is expressed in the units mpy (mills per year) or mm per year. This can be determined by any gravimetric method.
Prevention of Uniform Corrosion
One or more of the following methods can be adopted to prevent uniform corrosion.
- Cathodic Protection
- Corrosion Inhibitors
- Protective Coatings
- Selecting Proper Materials
Galvanic Corrosion
When dissimilar metals or alloys differing in their galvanic or corrosion potential are employed and if they are electrically shorted they induce this type of corrosion. The corrosion rate of the alloy with lower corrosion potential will be accelerated by that of higher corrosion potential.
Identification of Galvanic Corrosion
- The active metal is corroded
- Grooving of the interface
- Noble metal deposits from the stream
- Graphite lining or bricks
Prevention of Galvanic Corrosion
- Provide electrical insulation between the metal
- Choose alloys closer to the galvanic series
- Provide design in structure so as to make anodic to cathodic ratio extremely large.
- Coat both anode and cathodic areas. Otherwise, coat only the cathode.
- Protect the corroding metal with a sacrificial anode, which is anodic to the corroding metal.
Crevice Corrosion
Accelerated corrosion occurs if differential aeration exists due to crevice, metal joining (lap joints, flanges, etc.), or any deposits. Interestingly the location starving for oxygen is forced to become anodic and the region having free access to oxygen becomes the cathode.
Identification of Crevice Corrosion
- Rivets, flanges, and lap joints are attacked internally.
- Deposits such as corrosion products, organic deposits, growth of organisms, etc. cause corrosion.
- Improper drainage of vessels and pipelines causes an accelerated attack.
Prevention of Crevice Corrosion
- Avoid riveting, go in for welding
- Design for proper drainage
- For stainless steels, high Mo content (316, 317, and Hastelloy) reduces crevice corrosion
- Remove the deposits
- Use solid non-absorbent gaskets
Pitting Corrosion
Alloys in presence of certain ions (such as halides) are prone to pitting. The rate of penetration within the pit can be as high as one million times as compared to the surroundings.
Identification of Pitting Corrosion
- Pinholes
- Normally grow in the direction of gravity
- The alloy environment combination is likely to promote pitting
- Pitting has taken place along the inclusion
Prevention of Pitting Corrosion
- Eliminate the specific ions responsible for pitting (say halides in the case of SS)
- Choose an alloy resistant to pitting. In stainless steels, high Mo promotes resistance (haste alloys, duplex stainless steels)
- Mild steels serve better in a chloride environment than SS if a certain amount of uniform corrosion is tolerated. Monel has more resistance in this environment.
Selective Leaching (De-Zincification)
When noble and active elements form an alloy it results in the selective removal of the latter. As a consequence, the alloy loses its strength and fails prematurely. Cu-Zn alloys are well known wherein dezincification occurs if Zn content exceeds 15 wl. Similarly, there is de-nickelification, de-siliconation, de-cobaltification.
Identification of Leaching
- They give rise to plug and layered types of attack.
- Change in color (from yellow to brown in the cases of brasses)
- X-ray diffraction can sometimes reveal the selective removal of one element
- There can be a change in density in some cases.
Prevention of Leaching
- Addition of any one of the elements namely Sn, As, Sb and P
- Al addition reduces overall corrosion and to some extent dezincification.
Intergranular Corrosion
This type of corrosion occurs as a result of a selective attack of the grain boundaries when either the grain boundary becomes highly active or phases prone to selective attack are formed. Stainless steels, which are normally resistant to intergranular attack, when subjected to a heat treatment between 400-900 C become sensitive to intergranular corrosion (IGC). This range can vary depending on the composition of the alloy. This treatment is called sensitization treatment and the alloy is said to be sensitized. This is mainly due to the formation of Cr23C6 and the consequent grain boundary depletion. Welding, a common practice in fabrication causes such an IGC attack.
Identification of Intergranular Corrosion
- The attack of the alloy away from the weldment is called a heat-affected zone.
- Clear ditch type of attack along the grain boundary and consequent weakening seen at higher magnification.
Prevention of Intergranular Corrosion
- Choose low-carbon and extra-low-carbon stainless steels (such SS are 3041,3161,3171)
- Choose Ti or Ta and Nb containing alloys (321,347)
- Provide a solutions treatment to redissolve the carbides (1050 °C, 30 m)
Erosion Corrosion
When there is a relative movement of the corrosive environment with respect to the alloy it can lead to erosion-corrosion. Pipelines and heat exchangers are subjected to such a kind of failure.
Identification of Erosion Corrosion
- Attack at the bends in pipelines
- Grooves in the direction of liquid flow.
Prevention of Erosion Corrosion
- Reduce the velocity of the medium
- Choose hard materials
- Avoid sharp turns
- Provide hard coatings.
Cavitation Damage
Some variation in erosion-corrosion is cavitation damage. Here there is damage due to bubble formation and collapse when there is hydrodynamic variation in pressure difference along the line. At low-pressure water/liquid vaporizes. When the same is subjected to higher pressure bubble forms and subsequently implodes. This leads to plastic deformation and the formation of cavities as brought out in.
Fretting Damage
Moving/vibrating interfaces under load cause damage akin to wear called fretting damage. Here the relative movement is relatively small in angstroms. A typical failed surface under this process is brought out.
Stress Corrosion Cracking (SCC)
When there is a conjoint action of stress and environment. Stress corrosion cracking occurs (SCC). However, SCC is specific to an environment. The alloys are susceptible to SCC only when specific ions are present akin to pitting corrosion. In addition, the alloys fail only if the stress exceeds a threshold level below which they are safe.
Identification of SCC
- SCC in austenitic stainless steel is predominantly trans-granular in nature.
- Failure occurs by brittle mode.
- Ions promoting the SCC of that particular alloy must be present. Say Cl and O2 for austenitic SS and ammoniacal solution for Cu base alloys.
- If the alloy is sensitized it can promote an intergranular mode of cracking.
Prevention of SCC
- Select the alloy that is not susceptible to the environment.
- In the case of SS, control either Cr or O2 one can keep either one of the low.
- Apply load lower than the threshold stress.
- Provide compressive stresses by sandblasting or shot blasting.
- Avoid stress concentration.
Corrosion rates for a few materials
The following table provides some typical atmospheric corrosion rate values in mils/yr for some common materials
| Atmospheric Corrosion rates (mils/yr) | ||||
| Material | Rural | Industrial | Marine | Severe |
| A 242 type 1 | 0.05-0.16 | 0.06-0.60 | 0.17-0.37 | 0.83-2.20 |
| A 514 type B | 0.06-0.20 | 0.06-0.40 | 0.20-0.70 | 0.10-0.50 |
| A 514 type F | – | 0.34-1.60 | 0.30-1.00 | 0.53-0.70 |
| A 517 type B | 0.06-0.26 | 0.06-1.60 | 0.19-1.00 | 0.11-0.70 |
| A 588 Gr A | 0.10-0.24 | 0.14-0.47 | 0.25-0.80 | 3.05-4.1 |
| Structural Steel | 0.15-0.29 | 0.17-0.73 | 0.37-0.90 | 7.20-9.0 |
| Stainless Steel | Only Pitting, No general corrosion |
Few more useful Resources for you…
An Article on Forms of Corrosion
Corrosion under insulation: A Presentation
Corrosion Protection for Offshore Pipelines
Corrosion Monitoring Techniques & Surveys: A short Presentation
Guide for Coating Selection for External Bolting to Reduce Corrosion
Application of Anti-Corrosive Linings in Oil and Gas Industry
Anti-Corrosive Composites for Oil and Gas Industry
Piping Materials Basics
Piping Stress Analysis Basics
Piping Design and Layout Basics
